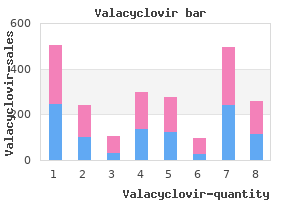
Valacyclovir
"Valacyclovir 500mg without prescription, zovirax antiviral tablets".
By: F. Rasul, M.A., M.D.
Associate Professor, University of Oklahoma College of Medicine
Modulation of high-voltageactivated calcium channels in dentate granule cells by topiramate hiv primo infection symptoms best valacyclovir 500mg. Topiramate protects against glutamate- and kainate-induced neurotoxicity in primary neuronalastroglial cultures antiviral side effects purchase valacyclovir canada. An overview of the preclinical aspects of topiramate: pharmacology hiv infection to symptom timeline valacyclovir 1000 mg mastercard, pharmacokinetics hiv infection symptoms skin purchase valacyclovir overnight delivery, and mechanism of action. Anticonvulsant activity of topiramate and phenytoin in a rat model of ischemia-induced epilepsy. Topiramate is both neuroprotective and antiepileptogenic in the pilocarpine model of status epilepticus [abstract]. Single-dose pharmacokinetics and effect of food on the bioavailability of topiramate, a novel antiepileptic drug. A study of topiramate pharmacokinetics and tolerability in children with epilepsy. Comparative single-dose pharmacokinetics of topiramate in elderly versus young men and women [abstract]. Tolerability and safety of topiramate as first-line monotherapy in 1,000 epilepsy patients [abstract]. A dose-comparison trial of topiramate as monotherapy in recently diagnosed partial epilepsy. Clinical experience with topiramate dosing and serum levels in children 12 years or under with epilepsy. Steady-state pharmacokinetics of topiramate and carbamazepine in patients with epilepsy during monotherapy and concomitant therapy. The steady-state pharmacokinetics of phenytoin (Dilantin Kapseals brand) and of Topamax (topiramate) in male and female epileptic patients on monotherapy, and during combination therapy. Comparison of the steady-state pharmacokinetics of topiramate and valproate in patients with epilepsy during monotherapy and concomitant therapy. Topiramate and lamotrigine pharmacokinetics during repetitive monotherapy and combination therapy in epilepsy patients. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. As first-line monotherapy in adults with newly or recently diagnosed epilepsy, 100 mg/day is an appropriate target dose to initially assess patient response. It appears the optimal starting dose in adults is 25 to 50 mg/day, with weekly or biweekly increases of 25 to 50 mg/day. As initial monotherapy in children, the recommended dose is 3 to 6 mg/kg/day, using a starting dose of 0. Cellular actions of topiramate blockade of kainate-evoked inward currents in cultured hippocampal neurons. Topiramate effects on excitatory amino acid-mediated responses in cultured hippocampal neurons: selective blockade of kainate currents [abstract]. Effects of topiramate on sustained repetitive firing and spontaneous recurrent seizure discharge in cultured hippocampal neurons. Effects of topiramate on sodiumdependent action-potential firing by mouse spinal cord neurons in cell culture. Inhibition of transient and persistent Na current fractions by the new anticonvulsant topiramate. Topiramate attenuates voltage-gated sodium currents in rat cerebellar granule cells. Frequency-dependent inhibition of neuronal activity by topiramate in rat hippocampal slices. Topiramate and phenytoin pharmacokinetics during repetitive monotherapy and combination therapy to epileptic patients. Topiramate: effective as monotherapy in dose-response study in newly diagnosed epilepsy [abstract].
Birth certificates are checked for additional information such as residency of the mother hiv infection symptoms in hindi cheap valacyclovir 1000 mg otc. Abstractors have specialized training and ongoing education to abstract medical records of potential cases hiv infection rates by country quality 1000 mg valacyclovir. Surveillance data are entered and maintained in a confidential electronic database hiv infection cd4 count purchase 1000 mg valacyclovir otc. Economic Impact on Massachusetts Estimating the economic impact of birth defects on the state of Massachusetts is challenging antiviral valacyclovir valacyclovir 500 mg cheap. The California Birth Defects Monitoring Program and the Metropolitan Atlanta Congenital Defects Program, using 1992 data, calculated the lifetime costs for families dealing with a baby with birth defects to be between $75,000 and $503,000 (Waitzman et al. Their estimated lifetime costs for a baby born with Spina Bifida would be $364,560 in 2003 dollars. Adjusting for inflation, the Massachusetts combined lifetime costs for babies born with 12 major structural birth defects were an estimated $122 million in 2003 dollars (see Technical Notes). These figures included direct costs of medical treatment, developmental services and special education, as well as indirect costs to society for lost wages due to early death or occupational limitations. Legislative Changes Regarding Birth Defects Surveillance In March 2002, the Massachusetts Legislature amended the state birth defects monitoring statute (Chapter 111, section 67E) to allow expansion of the surveillance system to capture diagnoses through age three. It also extends mandated reporters to include attending physicians, primary care and specialist physicians who may diagnose birth defects. These physicians will now have a statutory duty to report within 30 days of making such a diagnosis. The 2002-2003 Surveillance Report this report presents statewide data on the prevalence of birth defects in live births and stillbirths in Massachusetts during the years 2002 and 2003. The data are presented in combined form since the numbers are relatively small for individual defects. The first annual report presented Massachusetts data for birth defects for the year 1999. There was about a 12% increase in cases from 2000-2001 to 10 2002-2003 that is attributable to this improved case ascertainment. Unless otherwise indicated the report uses the term "births" to mean live births plus stillbirths. A stillbirth was defined as the delivery of a fetus that was not alive, and was greater than or equal to 20 weeks gestational age, or weighed at least 350 grams. Cases met the following criteria: the infant was live born or, the fetus was stillborn with a gestational age greater than or equal to 20 weeks or with a weight of at least 350 grams. The infant or fetus had a structural birth defect that met diagnostic criteria (see Birth Defects Codes and Exclusions by Defect Category in Appendices). Hospitals across the state submitted monthly discharge lists with birth defect diagnoses to the Center. If the infant or fetus had a birth defect that met the case definition criteria, detailed demographic and diagnostic information was recorded on a hospital reporting form. This information was entered into a confidential surveillance database for analysis. The Center has developed extensive procedures to guarantee the confidentiality of data and protect the privacy of families. These procedures uphold our ethical and legal obligations to safeguard confidentiality and fully comply with the strict requirements of state and federal laws. If the case had more than one defect within the same defect category, only one of these defects was counted in the category total. If the case had more than one defect in different defect categories, the case was listed in the total for each of these defect categories. Thus the counts in the defect categories presented in the prevalence tables represent the total number of defects, not the total number of cases with birth defects. In this report, maternal age and race/ethnicity are drawn from the birth certificate. Because birth certificate data are more accurate for these fields than fetal death records, analyses of maternal age and race/ethnicity are limited to live births. Prevalence is calculated as the number of birth defect cases born during the period 2002-2003 per 10,000 live births born during the same period. Prevalence tables include the number of cases found, the estimated prevalence rate per 10,000 live births, and the 95% confidence interval for that rate.
The recommended duration of nicotine patch therapy is 612 weeks hiv infection who generic 1000mg valacyclovir mastercard, with a tapering of the patch dose over that period; longer durations of patch therapy have not been found to be more effective (790) otc anti viral meds generic valacyclovir 1000mg online. The 4-mg dose is recommended for heavy smokers (>25 cigarettes/day) or more nicotine-dependent smokers (790 hiv primo infection symptoms proven valacyclovir 500mg, 802 hiv infection through cuts buy valacyclovir 1000mg online, 803). With nicotine gum, patients should be instructed to chew one piece of gum very slowly until a slight tingling or distinctive taste is noted, at which time the gum should be placed ("parked") between the cheek and gum until the taste or tingling is almost gone. Nicotine nasal spray and vapor inhaler systems provide faster delivery of nicotine than gum or lozenges, but still deliver nicotine more slowly and with lower peak nicotine levels than cigarettes. Nicotine vapor inhalers are cartridges of nicotine that are placed inside hollow cigarette-like plastic rods and produce a nicotine vapor (0. The recommended dose is 616 cartridges daily, with the inhaler being used ad libitum for about 12 weeks. Other agents There is also support for the use of nortriptyline and clonidine as treatments for nicotine dependence; however, given the number of other available treatments for which results are well validated, these should be viewed as second-line therapies. Nortriptyline may be particularly promising as a second-line nonnicotine pharmacotherapy, and its efficacy does not appear to depend on the presence of co-occurring depressive symptoms or major depressive disorder (795, 814). Clonidine Treatment of Patients With Substance Use Disorders 81 Copyright 2010, American Psychiatric Association. These therapies are typically provided as a multimodal package of several specific treatments and aim to provide patients with the skills to quit smoking and avoid smoking in high-risk situations. Cognitive coping skills may include identifying maladaptive thoughts, challenging them, and substituting more effective thought patterns to prevent a slip from becoming a relapse. The 6-month quit rates for behavioral therapies in general are typically 20%25%, or about twofold greater than quit rates with control conditions (824828). When brief interventions are used, patients are likely to have a greater number of quit attempts and a greater likelihood of success in smoking cessation (825, 826, 828). Specific types of behavioral therapy that have also been studied include contingency management, cue exposure, and "rapid smoking" aversion therapy; however, none of these are sufficiently well studied to support their use clinically. Little evidence is available that would support the use of physiological feedback. An inpatient stay may be an opportune time for initiating treatment for nicotine dependence because of the intensity of exposure to medical staff, diagnosis of medical conditions, and removal from usual smoking cues. It may therefore be helpful to include smoking cessation on the master treatment plan whenever relevant. Giving special off-ward privileges to allow patients to smoke or labeling offward passes as "smoking breaks" implicitly condones smoking (34, 866). Policies that provide breaks for smokers and nonsmokers on the same schedule may be preferable to policies that provide smokers with extra passes. Other recommendations for implementing a smoke-free unit are discussed in reviews (34, 866). Many patients are unaware of the valid symptoms of nicotine withdrawal and their time course; thus education about these can be helpful (34, 866). Although nicotine withdrawal symptoms are thought to be milder than alcohol withdrawal symptoms, there is substantial person-to-person variability so that some alcohol-dependent smokers have nicotine withdrawal symptoms that are more severe than their alcohol withdrawal symptoms (872). Further confounding the source of the increased symptoms is the fact that smoking cessation can cause dramatic increases in blood levels of some medications. Bupropion can also be used in inpatient settings given its fixed dosing, easy monitoring, and efficacy in reducing signs and symptoms of the withdrawal syndrome (158). Although the existing evidence does not show direct effects of psychosocial treatments on withdrawal symptoms, clinical experience suggests several strategies that may be useful for individuals in inpatient settings. Anger can be averted by temporarily avoiding interactions; insomnia can be decreased by improving sleep hygiene; weight gain can be combated by increasing activity; and distraction and activities aimed at keeping busy can be used to get through craving episodes. Support groups for those going smoke free and support from family and significant others for going smoke free can be helpful as well. Use of multiple substances There is strong evidence that rates of smoking are much higher in patients with a substance use disorder than in the general population (347). For example, in the National Epidemiologic Survey on Alcohol and Related Conditions, the 12-month prevalence of nicotine dependence is 34.
The fire was extinguished immediately but continued to flare up because the oil was still above its autoignition temperature antiviral breakfast buy discount valacyclovir line. An investigation determined that the thermocouple used by the oil bath temperature controller had fallen out of the oil bath antiviral natural generic valacyclovir 1000 mg without prescription. The controller hiv infection rates wiki cheap valacyclovir 500 mg fast delivery, responding to the false temperature drop reading hiv infection stories gay buy generic valacyclovir 500 mg line, continued to supply power to the bath, resulting in overheating and fire. Purchase or construct laboratory ovens with their heating elements and their temperature controls physically separated from their interior atmospheres. Small household ovens and similar heating devices usually do not meet these requirements and, consequently, should not be used in laboratories. With the exception of vacuum drying ovens, laboratory ovens rarely prevent the discharge of the substances volatilized in them into the laboratory atmosphere. The volatilized substances may also be present in sufficient concentration to form explosive mixtures with the air inside the oven (see Chapter 6, section 6. This hazard can be reduced by connecting the oven vent directly to an exhaust system. To avoid explosion, do not dry glassware that has been rinsed with an organic solvent in an oven until it has been rinsed again with distilled water. Potentially explosive mixtures can be formed from volatile substances and the air inside an oven. Do not mount mercury thermometers through holes in the tops of ovens with the bulb hanging into the oven. If a mercury thermometer is broken in an oven of any type, close the oven and turn it off immediately to avoid mercury exposure. Remove all mercury from the cold oven with the use of appropriate cleaning equipment and procedures (see Chapter 6, section 6. After removal of all visible mercury, monitor the heated oven in a laboratory chemical hood until the mercury vapor concentration drops below the threshold limit value. The first step of the analytical protocol called for ashing the sample in a muffle furnace. The technician loaded the furnace with four crucibles containing a total of approximately 110 g of polypropylene. At approximately 500 °C a fire erupted from the furnace, which was quickly extinguished. First, the technician had no experience with the analysis of polypropylene-containing samples and did not recognize that polypropylene begins to decompose at approximately 500 °C to low-molecular-weight olefins. Second, the amount of organic matter placed in the furnace in the form of the polypropylene samples was significantly more than that in the usual paint samples. Laboratory hot plates are often used when solutions are to be heated to 100 °C or higher and the inherently safer steam baths cannot be used as the source of heat. As previously noted, use only hot plates that have completely enclosed heating elements in laboratories. Although almost all laboratory hot plates currently sold meet this criterion, many older ones pose an electrical spark hazard arising from either the on/off switch located on the hot plate, the bimetallic thermostat used to regulate the temperature, or both. Normally, these two spark sources are located in the lower part of the hot plate in a region where any heavier-than-air and possibly flammable vapors evolving from a boiling liquid on the hot plate would tend to accumulate. In principle, these spark hazards are alleviated by enclosing all mechanical contacts in a sealed container or by using solid-state circuitry for switching and temperature control. However, in practice, such modifications are difficult to incorporate into many of the hot plates now in use. In addition to the spark hazard, old and corroded bimetallic thermostats in these devices can eventually fuse shut and deliver full continuous current to a hot plate. This risk can be avoided by wiring a fusible coupling into the line inside the hot plate. If the device does overheat, the coupling will melt and interrupt the current (see section 7. On many brands of combined stirrer/hot plates, the controls for the stirrer and temperature control are not easily differentiated. A fire or explosion may occur if the temperature rather than the stirrer speed is increased inadvertently. As long as the fiberglass coating is not worn or broken and no water or other chemicals are spilled into the mantle (see section 7. They are normally fitted with a male plug that fits into a female receptacle on an output line from a variable autotransformer.
The European Union (European Chemicals Bureau 2003) estimated that the total daily intake from these exposures was 54 hiv infection without penetration order valacyclovir 1000mg mastercard. Emissions estimates for California indicated that gasoline evaporation and engine exhaust accounted for 44% of total naphthalene emissions into ambient air; diesel exhaust was estimated to contribute another 9% of the total (Lu et al hiv infection rate condom valacyclovir 1000mg. Other major emissions sources hiv infection per year buy discount valacyclovir 1000mg on line, including asphalt and a large number of consumer products hiv infection rates us order valacyclovir 500mg visa, contributed 15% of emissions. The first step, oxidation via cytochrome P450 monooxygenases, produces an unstable 1,2-epoxide that can convert nonenzymatically to 1-naphthol. The epoxide can also be * Naphthalene was not included in the survey of indoor exposures and is thus not listed in the indoor exposure table in Appendix D. The naphthol and the diol compounds can be further oxidized to form quinones, which, along with the epoxides, represent reactive toxic metabolites that can bind to macromolecules. Mice are the most sensitive (of the species tested) to the toxicity of inhaled naphthalene. Maximal rates of naphthalene metabolism measured in human lung microsomes are about 10 to 100 times lower than those in mice. These data suggest that the respiratory tract of humans is likely to be much less sensitive than that of mice to the toxicity of inhaled naphthalene (Figure 20). In nonciliated bronchiolar epithelial cells (Clara cells) isolated from the lungs of naphthalene-treated mice, covalent binding of 1,2-naphthoquinone to protein was reported. Treatment with the glutathione depletor diethylmaleate before naphthalene exposure decreased water-soluble naphthalenemetabolite formation by 48% yet increased naphthalene protein adducts by 193% (Phimister et al. In mice, glutathione depletion in Clara cells seems to be a determinant of the specific pulmonary toxicity of naphthalene. Plopper and colleagues (2001) have investigated early events in naphthalene-induced acute Clara-cell toxicity. Two hours after intraperitoneal injection of 200 mg/kg body weight naphthalene, they observed the highest glutathione depletion in the most susceptible distal bronchioles. Although severe glutathione depletion can lead to apoptosis and cell proliferation (Rahman et al. Whereas these disruptive cellular changes seemed to be reversible after recovery of glutathione levels, they persisted after naphthalene exposure. Studies published recently by Lee and colleagues (2005) showed that the olfactory regions of the nasal septum and ethmoturbinates metabolize naphthalene at higher rates than the non-olfactory mucosa of the nasal septum. The regions of the nasal mucosa with high rates of naphthalene metabolism were the ones injured by inhaled or systemically administered naphthalene. These sites are also the location of high concentrations of cytochrome P450 enzymes, capable of oxidizing naphthalene to its reactive forms, and of the cellular systems with the highest rate of glutathione depletion (see above). Exposure of mice (but not rats) for 4 hours to concentrations as low as 10 mg/m3 naphthalene resulted in detectable injury to Clara cells. In rats, after repeated exposures to 52 mg/m3, non-neoplastic lesions were found in the olfactory and respiratory epithelia of the nose. Interestingly, if naphthalene is administered to mice intraperitoneally, the injury site is still the epithelial cells of the respiratory tract. In mice exposed to cigarette smoke, recovery from naphthalene-induced injury to the bronchiolar epithelium was impaired. Clara cells of neonatal mice were more sensitive than those of adult mice to the cytotoxic effects of naphthalene. In rats and rabbits, repeated oral administration of naphthalene is known to cause cataract formation at doses of 700 mg/kg body weight/day and above. In isolated mouse Clara cells, 1,4-naphthoquinone and naphthalene 1,2-oxide were more toxic than naphthalene. Reproductive and Developmental Effects No studies of the effects of naphthalene exposure on the fertility of animals have been reported. Changes in the reproductive organs have not been detected in repeateddose studies (including chronic-inhalation studies), and there are no available data on the effects of naphthalene exposure on reproductive function. In rats and rabbits exposed to naphthalene by gavage, signs of toxicity were observed in pregnant females. In mice exposed by 110 Naphthalene gavage, signs of toxicity were found both in pregnant females (increased mortality and reduced weight gain) and in fetuses (reduced number of live pups per litter).
Purchase 1000 mg valacyclovir visa. Stage III: Tertiary Stage of HIV.