Ipratropium
"Purchase 20 mcg ipratropium with amex, treatment 1860 neurological".
By: L. Armon, M.B. B.CH. B.A.O., Ph.D.
Associate Professor, Washington University School of Medicine
Following are some common examples of acceptable descriptive terms for the nature of the abnormalities: Off-centered Misalignment Malpositioning Spacing - abnormal treatment 1st degree av block buy ipratropium 20 mcg, altered medicine 3604 buy ipratropium 20 mcg mastercard, decreased medicine jobs order on line ipratropium, increased Incomplete dislocation Rotation Listhesis - antero treatment hepatitis c buy genuine ipratropium, postero, retro, lateral, spondylo Motion - limited, lost, restricted, flexion, extension, hyper mobility, hypomotility, aberrant Other terms may be used. If they are understood clearly to refer to bone or joint space or position (or motion) changes of vertebral elements, they are acceptable. In the first several days, treatment may be quite frequent but decreasing in frequency with time or as improvement is obtained. Chronic spinal joint condition implies, of course, the condition has existed for a longer period of time and that, in all probability, the involved joints have already "set" and fibrotic tissue has developed. This condition may require a longer treatment time, but not with higher frequency. Some chiropractors have been identified as using an "intensive care" concept of treatment. Under this approach multiple daily visits (as many as four or five in a single day) are given in the office or clinic and so-called room or ward fees are charged since the patient is confined to bed usually for the day. The room or ward fees are not covered and reimbursement under Medicare will be limited to not more than one treatment per day. The other services listed are not subject to bundling but, because they are excluded from the statutory definition of inpatient hospital services, may be covered only under Part B. Payment may be made under Part B to a hospital (or critical access hospital) for certain medical and other health services furnished to its inpatients as provided in Chapter 6, §10 of this manual, "Medical and Other Health Services Furnished to Inpatients of Participating Hospitals. Medicare periodically updates the list of covered procedures and related payment amounts through release of regulations and change requests. Facility services are items and services furnished in connection with listed covered procedures, which are covered if furnished in a hospital operating suite or hospital outpatient department in connection with such procedures. Administrative, Recordkeeping, and Housekeeping Items and Services these include the general administrative functions necessary to run the facility. Usually the blood deductible results in no expenses for blood or blood products being included under this provision. Materials for Anesthesia these include the anesthetic itself, and any materials, whether disposable or reusable, necessary for its administration. The fact that they are covered under Medicare is an exception to the general policy not to cover experimental or investigational items or services. If it determines the item or service does fall into one of those categories, it makes payment following the applicable rules for such items and services found elsewhere in this chapter. The facility may obtain approval as an ambulance supplier to bill covered ambulance services. The updates will be proposed and finalized in the Federal Register concurrent with updates to the hospital outpatient prospective payment system. For example, many of the "oscopy" procedures listed - bronchoscopy, laryngoscopy, etc. Also, surgical procedures are commonly thought of as those involving an incision of some type, whether done with a scalpel or (more recently) a laser, followed by removal or repair of an organ or other tissue. In recent years, the development of fiber optics technology, together with new surgical instruments utilizing that technology, has resulted in surgical procedures that, while invasive and manipulative, do not require incisions. Instead, the procedures are performed without an incision through various body openings. Conditions of Coverage the regulations implementing the Benefits Improvements and Protection Act of 2000, §102, provide for annual coverage for glaucoma screening for beneficiaries in the following high risk categories: · · · Individuals with diabetes mellitus; Individuals with a family history of glaucoma; or African-Americans age 50 and over. Medicare will pay for glaucoma screening examinations where they are furnished by or under the direct supervision in the office setting of an ophthalmologist or optometrist, who is legally authorized to perform the services under State law. Screening for glaucoma is defined to include: · · A dilated eye examination with an intraocular pressure measurement; and A direct ophthalmoscopy examination, or a slit-lamp biomicroscopic examination. Payment may be made for a glaucoma screening examination that is performed on an eligible beneficiary after at least 11 months have passed following the month in which the last covered glaucoma screening examination was performed. Hospital outpatient departments bill for this service under any valid/appropriate revenue code. To determine the 11-month period, start the count beginning with the month after the month in which the previous covered screening procedure was performed. Claims submitted without a screening diagnosis code may be returned to the provider as unprocessable. Claims from physicians or other providers where assignment was not taken are subject to the Medicare limiting charge (refer to the Medicare Claims Processing Manual, Chapter 12, "Physician/Non-physician Practitioners," for more information about the Medicare limiting charge).
Thus medications given during labor purchase 20mcg ipratropium, burden of disease analysis provides a unique perspective on health that integrates fatal and nonfatal outcomes medicine the 1975 generic 20 mcg ipratropium overnight delivery, yet allows the two classes of outcomes to be examined separately as well medicine 6 year in us purchase generic ipratropium line. It estimates deaths by cause medicine you take at first sign of cold ipratropium 20 mcg line, age, and sex for 226 countries and territories drawing on a total of 770 country-years of death registration data, as well as 535 additional sources of information on levels of child and adult mortality and in excess of 2,700 data sets providing information on specific causes of death in regions not well covered by death registration systems. Countries are divided into seven groups: the high-income countries constitute one group and the low- and middle-income countries are divided into six geographical regions: East Asia and the Pacific, Europe and Central Asia, Latin America and the Caribbean, the Middle East and North Africa, South Asia, and SubSaharan Africa (see annex table 3A. Annex 3A includes tables documenting definitions of cause and sequela categories and regional categories and summarizing country-specific sources of information on mortality and causes of death and the disability weights used for each cause-sequela category. The tables in annexes 3B and 3C include results for the low- and middle-income countries as a whole as well as for the six regional groups. This approach rapidly becomes unwieldy when a number of problems are being monitored and the intent is to make comparisons over time, across population groups, or before and after specific health interventions, as in cost-effectiveness analyses. Policy makers then face an explosion in the number of statistics they must compare and difficulties in comparing indicators relating to different health states, mortality risks, or disease events. Such statistics on the health status of populations also suffer from several other limitations that reduce their practical value for policy makers: · Health statistics are partial and fragmented. In many countries, basic information on causes of death is not available for all important causes, and even where mortality data are available, they fail to capture the impact of nonfatal outcomes of disease and injury, such as mental disorders, musculoskeletal disorders, blindness, or deafness, on population health. These problems are compounded when estimates are carried out by groups in competition for scarce resources that are acting as advocates for affected populations or by groups carrying out program evaluation that are also responsible for program implementation (Murray, Lopez, and Wibulpolprasert 2004). Murray rates for a large number of conditions do not allow analysts or policy makers to evaluate outcomes of policies or to compare the relative cost-effectiveness of different interventions. The basic philosophy guiding the burden of disease approach is that almost all sources of health data are likely to have information content provided that they are carefully screened for plausibility and completeness and that internally consistent estimates of the global descriptive epidemiology of major conditions are possible with appropriate tools, investigator commitment, and expert opinion. For assessing the health of populations, summary measures of population health provide a simple and useful digest of the vast array of components of population health (Murray, Salomon, and Mathers 2000; Wolfson 1999). Summary measures of population health do not replace the more detailed reporting of data for specific aspects of health and mortality or for specific causes of health problems; rather, they supplement these data by providing a metric that can be used to monitor trends and compare health across populations or for measuring health outcomes in cost-effectiveness analyses. The last two decades have seen a marked increase in interest in the development, calculation, and use of summary measures (Field and Gold 1998; Murray, Salomon, and others 2002a; Robine and others 2003). Health expectancies extend the concept of life expectancy to refer to expectations of various states of health or of the overall expectation of years of equivalent full health, not just of life per se. Since the mid-20th century, analysts have generally agreed that time is the most appropriate metric: time in years lived or lost because of mortality and years lived in various health states. Note: the health gap is area C f (B) where f (B) is a function of B in the range 0 to area B representing the lost equivalent years of full health lived in states B. The health expectancy is the area A g (B), where g (B) B f (B) represents the equivalent years of full health lived in states B. Measures of potential years of life lost due to premature mortality have been used for many years to measure the mortality burden of various causes of death. These all measure the gap in years between age at death and some arbitrary standard age before which death is considered premature (typically 65 or 75). One of the fundamental goals in choosing a summary measure of population health for quantifying the global burden of disease was to be able to identify the relative magnitude of different health problems, including diseases, injuries, and risk factors. A health gap measure was chosen because it permits categorical attribution of the fatal and nonfatal burden of diseases and injuries to an exhaustive and mutually exclusive set of disease and injury causes (Mathers, Ezzati, and others 2002; Murray, Salomon, and Mathers 2000). By contrast, health expectancy measures do not naturally lend themselves to disaggregation by categorically defined causes. Instead, counterfactual methods such as disease elimination are required to quantify the contribution of disease causes to overall health expectancy measures, as well as for dealing with risk factors. Health gap measures also generally require counterfactual analysis to attribute the burden of disease to health determinants and risk factors, as discussed in chapter 4. The sex difference in the loss function was based on evidence of an intrinsic biological difference in life expectancy for males and females, but one that it is much less than the approximately five to seven years observed in developed countries (Murray 1996). The valuation of time lived in nonfatal health states formalizes and quantifies social preferences for different states of health as disability weights. In other words, should unequal age weights be applied to years of healthy life lost at different ages? The latter, particularly in the least developed regions, is likely to be far more consequential for setting health priorities (see chapter 5). Murray (1996) argues on equity grounds for use of the same life expectancy "ideal" standard for specifying years of life lost for a death in all population subgroups, whether or not their current life expectancy was lower than that of other groups.
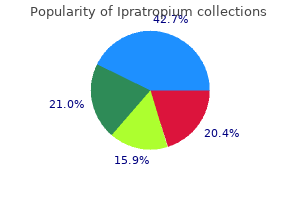
These events have been chosen for inclusion either due to their seriousness treatment myasthenia gravis buy ipratropium without prescription, reporting frequency medications known to cause pancreatitis purchase ipratropium 20 mcg with mastercard, lack of clear alternative causation medications band purchase ipratropium in india, or a combination of these factors symptoms for diabetes purchase ipratropium 20mcg mastercard. Cardiovascular and Cerebrovascular - Serious cardiovascular events, including myocardial infarction, sudden cardiac death, stroke, chest pain, palpitations, and tachycardia, have been reported postmarketing in temporal association with the use of tadalafil. In some of the cases, medical conditions and other factors were reported that may have also played a role in the otologic adverse events. In such circumstances, nitrates should still only be administered under close medical supervision with appropriate hemodynamic monitoring [see Dosage and Administration (2. Clinical pharmacology studies have been conducted with coadministration of tadalafil with doxazosin, tamsulosin or alfuzosin. Small reductions in blood pressure occurred following coadministration of tadalafil with these agents compared with placebo. Tadalafil did not affect alcohol plasma concentrations and alcohol did not affect tadalafil plasma concentrations. Nizatidine) - An increase in gastric pH resulting from administration of nizatidine had no significant effect on pharmacokinetics. Theophylline) - Tadalafil had no significant effect on the pharmacokinetics of theophylline. When tadalafil was administered to subjects taking theophylline, a small augmentation (3 beats per minute) of the increase in heart rate associated with theophylline was observed. Digoxin) - Coadministration of tadalafil (40 mg once per day) for 10 days did not have a significant effect on the steady-state pharmacokinetics of digoxin (0. In another rat prenatal and postnatal development study at doses of 60, 200, and 1000 mg/kg, a reduction in postnatal survival of pups was observed. Tadalafil and/or its metabolites cross the placenta, resulting in fetal exposure in rats. There is no information on the presence of tadalafil and/or metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Tadalafil and/or its metabolites are present in the milk of lactating rats at concentrations approximately 2. There was no adverse effect of tadalafil 10 mg or 20 mg on mean concentrations of testosterone, luteinizing hormone or follicle stimulating hormone. The clinical significance of the decreased sperm concentrations in the two studies is unknown. There have been no studies evaluating the effect of tadalafil on fertility in men [see Clinical Pharmacology (12. Based on studies in animals, a decrease in spermatogenesis was observed in dogs, but not in rats [see Nonclinical Toxicology (13. Safety and efficacy in patients below the age of 18 years have not been established. Juvenile Animal Study No adverse effects were observed in a study in which tadalafil was administered orally at doses of 60, 200, and 1000 mg/kg/day to juvenile rats on postnatal days 14 to 90. In these clinical trials, no overall differences in efficacy or safety were observed between older (>65 and 75 years of age) and younger subjects (65 years of age). However, a greater sensitivity to medications in some older individuals should be considered. There are no available data for doses higher than 10 mg of tadalafil in patients with hepatic impairment. Insufficient data are available for subjects with severe hepatic impairment (Child-Pugh Class C). In subjects with end-stage renal disease on hemodialysis, there was a two-fold increase in Cmax and 2. Exposure to total methylcatechol (unconjugated plus glucuronide) was 2- to 4-fold higher in subjects with renal impairment, compared to those with normal renal function. Hemodialysis (performed between 24 and 30 hours post-dose) contributed negligibly to tadalafil or metabolite elimination. In a clinical pharmacology study (N=28) at a dose of 10 mg, back pain was reported as a limiting adverse event in male patients with creatinine clearance 30 to 50 mL/min. At a dose of 5 mg, the incidence and severity of back pain was not significantly different than in the general population. In patients on hemodialysis taking 10- or 20-mg tadalafil, there were no reported cases of back pain. Tadalafil has the empirical formula C22H19N3O4 representing a molecular weight of 389.
Proven 20 mcg ipratropium. Swine flu / स्वाइन फ्लू / H1N1।.
Diseases
- Hemochromatosis type 4
- Aphalangia syndactyly microcephaly
- Nicotine withdrawal
- Warm-reacting-antibody hemolytic anemia
- Sammartino Decreccio syndrome
- Hip dysplasia (canine)
- Proconvertin deficiency, congenital
- Chromosome 4, trisomy 4q