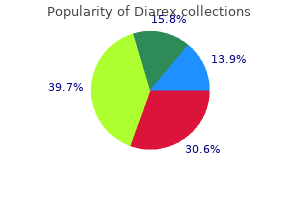
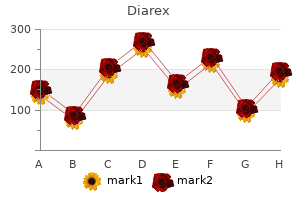
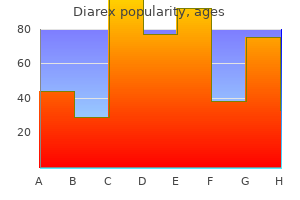
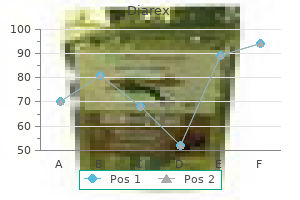
Diarex
"Trusted diarex 30caps, gastritis diet эротика".
By: N. Mazin, M.B. B.CH. B.A.O., Ph.D.
Clinical Director, Cooper Medical School of Rowan University
Special monthly compensation should be assigned concurrently in these cases whenever records are adequate to establish entitlement gastritis and gas buy diarex canada. The following ratings may be assigned gastritis upper left abdominal pain generic diarex 30caps line, in lieu of ratings prescribed elsewhere superficial gastritis definition buy diarex 30 caps without a prescription, under the conditions stated for disability from any disease or injury gastritis blood test buy diarex with a mastercard. The prestabilization rating is not to be assigned in any case in which a total rating is immediately assignable under the regular provisions of the schedule or on the basis of individual unemployability. The prestabilization 50-percent rating is not to be used in any case in which a rating of 50 percent or more is immediately assignable under the regular provisions. Rating Unstabilized condition with severe disability- Substantially gainful employment is not feasible or advisable. Unhealed or incompletely healed wounds or injuries- Material impairment of employability likely. Prestabilization ratings are for assignment in the immediate postdischarge period. However, prestabilization ratings may be changed to a regular schedular total rating or one authorizing a greater benefit at any time. In each prestabilization rating an examination will be requested to be accomplished not earlier than 6 months nor more than 12 months following discharge. A total disability rating (100 percent) will be assigned without regard to other provisions of the rating schedule when it is established that a serviceconnected disability has required hospital treatment in a Department of Veterans Affairs or an approved hospital for a period in excess of 21 days or hospital observation at Department of Veterans Affairs expense for a serviceconnected disability for a period in excess of 21 days. A temporary release which is approved by an attending Department of Veterans Affairs physician as part of the treatment plan will not be considered an absence. An authorized absence of 4 days or less which results in a total of more than 8 days of authorized absence during the first 21 days of hospitalization will be regarded as the equivalent of hospital discharge effective the ninth day of authorized absence. If service connection for the disability under treatment is granted after hospital admission, the rating will be from the first day of hospitalization if otherwise in order. Particular attention, with a view to proper rating under the rating schedule, is to be given to the claims of veterans discharged from hospital, regardless of length of hospitalization, with indications on the final summary of expected confinement to bed or house, or to inability to work with requirement of frequent care of physician or nurse at home. A total disability rating (100 percent) will be assigned without regard to other provisions of the rating schedule when it is established by report at hospital discharge (regular discharge or release to non-bed care) or outpatient release that entitlement is warranted under paragraph (a) (1), (2) or (3) of this section effective the date of hospital admission or outpatient treatment and continuing for a period of 1, 2, or 3 months from the first day of the month following such hospital discharge or outpatient release. When the evidence is inadequate to assign a schedular evaluation, a physical examination will be scheduled and considered prior to the termination of a total rating under this section. The total rating will be followed by an open rating reflecting the appropriate schedular evaluation; where the evidence is inadequate to assign the schedular evaluation, a physcial examination will be scheduled prior to the end of the total rating period. Weakness is as important as limitation of motion, and a part which becomes painful on use must be regarded as seriously disabled. A little used part of the musculoskeletal system may be expected to show evidence of disuse, either through atrophy, the condition of the skin, absence of normal callosity or the like. In considering the residuals of injury, it is essential to trace the medical-industrial history of the disabled person from the original injury, considering the nature of the injury and the attendant circumstances, and the requirements for, and the effect of, treatment over past periods, and the course of the recovery to date. The duration of the initial, and any subsequent, period of total incapacity, especially periods reflecting delayed union, inflammation, swelling, drainage, or operative intervention, should be given close attention. This consideration, or the absence of clear cut evidence of injury, may result in classifying the disability as not of traumatic origin, either reflecting congenital or developmental etiology, or the effects of healed disease. The importance of complete medical examination of injury cases at the time of first medical examination by the Department of Veterans Affairs cannot be overemphasized. When possible, this should include complete neurological and psychiatric examination, and other special examinations indicated by the physical condition, in addition to the required general and orthopedic or surgical examinations. When complete examinations are not conducted covering all systems of the body affected by disease or injury, it is impossible to visualize the nature and extent of the service connected disability.
Studies of combined cohorts comprising many of the workers included in individual studies have been carried out in the United Kingdom and the United States gastritis hot flashes cheap diarex uk, as well as studies of all workers included in the national dose registries in Canada gastritis diet евроспорт purchase diarex from india, Japan gastritis diet quality cheap 30caps diarex fast delivery, and the United Kingdom gastritis diet контакт purchase diarex canada. The latest analysis included 35,933 workers, followed until the end of 1986 (Gilbert and others 1993a). A study of workers employed in one of 15 commercial nuclear power facilities was also conducted (Howe and others 2004). The latest publication covers follow-up for mortality until the end of December 1992. About 25% of these were nuclear industry workers, but detailed results were not presented for this group. The study included 45,468 workers monitored for more than 1 year between 1957 and 1994. Consequently the study had little power to assess possible health effects of occupational ionizing radiation exposure; in particular, the test for trend for all cancers had a one-sided p-value of 0. Overall, 95,673 workers employed between 1943 and 1988 in one of the participating facilities were included. Characteristics of Studies of Nuclear Industry Workers In the majority of the studies listed above, study subjects are defined as workers employed in the nuclear industry for whom detailed individual external dose estimates were available. The number of workers and person-years of follow-up in the major studies are listed in Table 8-2. In general, exposure in most of these cohorts was predominantly to low levels of external radiation (X- and -rays and some neutrons). Internal contamination (through inhalation, ingestion, skin absorption, or wounds) by tritium, plutonium, uranium, and other radionuclides occurred in some subgroups of workers. Assessment of Exposure to Radiation Control of radiation dose to workers in occupational settings is achieved by demarcating radiation levels in work areas, conducting routine radiation monitoring. Individual monitoring at its simplest consists of assigning radiation-sensitive dosimeters to each worker. Dosimeters, which consist of one or more of ionization chambers, photographic film, luminescent phosphors, or electronic devices, are worn by workers while they are present in designated radiation areas. Dosimeters are normally placed on the chest, and it is usually assumed that the measured radiation dose is representative of the whole-body dose. In nearly all cases, dosimeters are sensitive to the penetrating photon radiation of intermediate (>100 keV) to higher photon. Specialized dosimeters and calibration methods are generally needed to measure accurately the dose from low-energy photons, beta, or neutron radiation present in some occupational environments. Monitoring for the intake of radioactive material is performed by bioassay, by whole-body in vivo counting, or by wearing personal air samplers. In most of the facilities that have been the object of the epidemiologic studies described above, measurements of dose to individuals have generally been recorded on a routine basis using the available dosimetry technology. Occupational radiation dose data constitute the most complete and detailed information currently available to researchers for studying the carcinogenic effects of low-dose, Copyright National Academy of Sciences. They are generally presented in the form of annual summaries of doses from different types of radiation (penetrating photons, beta, and where appropriate and measured, tritium and neutrons). These data were, however, compiled to monitor worker exposure for compliance with radiation protection guidelines, which have changed over time, and not specifically for epidemiologic purposes. Consequently, detailed examination of dosimetry practices, including sources and magnitude of errors, is important in considering whether sufficiently accurate and precise estimates of dose can be obtained for use in an epidemiologic study. Information on internal contamination with radionuclides other than tritium is generally sparse, particularly in early years, and consists of information on the fact of monitoring or on a percentage of the annual limit of intake. Very few studies have attempted to reconstruct individual doses from nuclides other than tritium. One exception is the study of Sellafield workers in the United Kingdom, where efforts have been made to reconstruct plutonium exposures (Omar and others 1999). In high-dose studies, the majority of excess deaths from cancer have been demonstrated in subjects exposed to doses of at least 1 Sv. Doses received by employees of nuclear industry facilities are considerably lower.
The resulting lower confidence limit was then compared to the target agreement rate of 0 gastritis diet шрек order diarex with a visa. Table 4: Inter-Site Descriptive Statistics for the Cologuard Score Sample Negative Stool Pool High Negative Stool Pool Low Positive Stool Pool N 387 394 393 Mean 9 gastritis hiv symptom generic diarex 30 caps on line. A total of 22 Cologuard runs were performed during the study gastritis diet чернобыль diarex 30 caps without prescription, in which each operator team performed 11 complete runs gastritis meal plan order genuine diarex online, with each run requiring 2 shifts to complete. Each run involved 12 samples, including six replicates of each of the high negative and low positive stool samples. For the molecular assay component of Cologuard, the stool sample types were prepared by combining characterized residual stool samples available to Exact Sciences. For each sample in the panel, there were 24 sample results per lot and 72 sample results for the entire study. Across the seven samples in the panel, there were 168 results per lot, and 504 results for the entire study. Percent positive results for the Cologuard Score were analyzed across lots and for lot to lot. Descriptive statistics were calculated for all marker/sample combinations, including median, mean, mean upper and lower 95% confidence intervals, standard deviation, and coefficient of variation values. Robustness the Cologuard performance was assessed in response to defined variable factors (see below) at specific steps in the test procedure, using both the molecular assay and hemoglobin assay components of Cologuard. The processing steps analyzed in this study are the steps at which operator variability or error are most likely to occur. Cologuard Molecular Assay Robustness Results when these various factors were introduced into the processing steps were compared to the expected results for a positive stool sample, a control sample with high levels of mutation and methylation markers, and a control sample with moderate levels of mutation and methylation markers. Analysis of these samples assumed a hemoglobin value of zero, when calculating overall Cologuard score. Cologuard Hemoglobin Assay Robustness Results when these factors were introduced into the processing steps were compared to the expected results for a stool sample with a known level of endogenous hemoglobin and a high and low control sample with high and low levels of hemoglobin. Analysis of these results involved comparing the resulting hemoglobin concentration with the expected hemoglobin concentration. Factors tested include the following: Time between steps during plate preparation; Incubation times for antibodies and substrates; and Time between steps during plate reading phase. Results for the hemoglobin assay component of Cologuard showed that substrate incubation time had a detectable effect on assay performance. No interference with the molecular assay component of Cologuard was observed for any of the tested substances. Carry-over and Cross-contamination Cologuard Testing Carry-over Evaluation Sequential runs of high positive and negative samples were used to evaluate carry-over contamination for each assay component of Cologuard. Testing of the molecular assay and hemoglobin assay components was conducted in two separate studies. A total of 43 high positive samples and 3 run controls were used in each high positive run. For the hemoglobin assay, the testing involved two consecutive runs of high positive hemoglobin samples, composed of 100,000 ng/mL hemoglobin, followed by a run of negative samples composed solely of the protein preservative solution from the hemoglobin sample collection tube. The high positive samples consisted of a hemoglobin level that is much higher than the quantitative range of the assay, which identifies all samples >500 ng/mL as greater than the maximum range of the assay. For the high positive runs, a total of 86 high positive hemoglobin samples were used. In each run, the signal obtained on the controls was utilized to ensure the validity of the run. Results from the molecular assay and hemoglobin assay carry-over analyses demonstrated that there was no carry-over in the Cologuard assay. Cross-contamination Evaluation Cross-contamination testing of Cologuard was based on a checkerboard study design, alternating high positive and negative samples, to evaluate the potential for contamination from the positive to the negative samples within a run. One run was performed and samples were processed using the Cologuard molecular process from the semi-automated front end sample processing through the automated processing.
Syndromes
- Sore throat
- Stress or anxiety
- Infection (a slight risk any time the skin is broken)
- Your doctor or nurse may ask you to use enemas or laxatives to clear out your intestines. They will give you instructions.
- Pap smear (may raise a suspicion for endometrial cancer, but does not diagnose it)
- Ask your doctor which drugs you should still take on the day of the surgery.
- Interrupts or intrudes on others (butts into conversations or games)
- Congenital dyserythropoietic anemia
- Parathyroid disease
Among viral diseases gastritis pediatric symptoms order cheap diarex on-line, Herpesvirus stomatitis gastritis liquid diet generic diarex 30 caps with amex, shingles and cytomegalovirus predominate gastritis symptoms vs. heart attack diarex 30 caps cheap. These diseases may be acquired from live infected animals erythematous gastritis definition discount diarex american express, dead animals, or from contaminated inanimate objects where the virus persists despite heat, cold or desiccation. Ancillary studies that may be used include electron microscopy examination, 96 immunohistochemistry and virologic culture. Infectious Complications among 620 Consecutive Heart Transplant Patients at Stanford University Medical Center. Cutaneous pseudolymphoma in association with molluscum contagiosum in an elderly patient. Perniosis may be associated with systemic lupus erythematosus or antiphospholipid antibodies. There are only rare recognized genetic forms of chilblain associated with lupus erythematosus. Clinical Features this is a reaction to cold, and is seen in outdoor activities such as horse riding and other outdoor winter pursuits. Familial Chilblain Lupus, a Monogenic Form of Cutaneous Lupus Erythematosus, Maps to Chromosome 3p. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases Hum Pathol 1997;4:478-84. Perniosis: clinical and histopathological analysis Am J Dermatopathol 2010;32:19-23. The provided clinical differential diagnosis on the pathology requisition sheet was nevus versus pigmented basal cell carcinoma versus melanoma. Histologically combined melanocytic nevi show oval and dendritic shaped melanocytes and melanophages admixed with nests of round and oval melanocytes. Also known as a sclerosing melanocytic nevus, these lesions show dermal sclerosis in the deeper aspect of the nevus. Nuclear atypia of the dermal spindled cells, features that can be seen in desmoplastic and spindle cell melanoma, are not evident. There is focal dermal fibrosis seen consistent with the history of a prior procedure. Although this lesion has focal features of a persistent or recurrent melanocytic nevus consistent with the provided clinical history, the spiondle cell proliferation consistent with a perineurioma component is incompatible with a routine recurrent nevus. Question 10 Which is the combination of immunohistochemical markers that will highlight the spindled cells and be most helpful in confirming the diagnosis Although the spindled cells in the dermis can express S100, S-100 is also a marker of melanocytes and thus does not help in confirming the nerve sheath component of this lesion. Although the spindled cells in the dermis can express S-100, S-100 is also a marker of melanocytes and thus does not help in confirming the nerve sheath component of this lesion. S-100 and Sox-10 are expressed by both melanocytic and neural tumors and would thus not help in the differentiation. Clinical Features 100 Melanocytic nevi with nerve sheath differentiation are a unique subset of tumors that display both conventional melanocytic nevus morphology and a distinct spindled cell population enmeshed in a delicate collagenous or myxoid stroma akin to benign nerve sheath tumors. Histologic features Microscopically, melanocytic nevi with nerve sheath differentiation have been divided into three groups: 1. The relationship between melanocytes and peripheral nerve sheath cells (Part I): melanocytic nevus (excluding so-called "blue nevus") with peripheral nerve sheath differentiation. Hybrid schwannoma/perineuroma:: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors.
Buy diarex 30caps on-line. Signs and Symptoms of Irritable Bowel Syndrome.